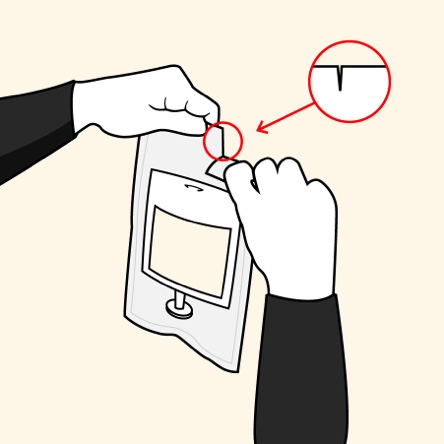
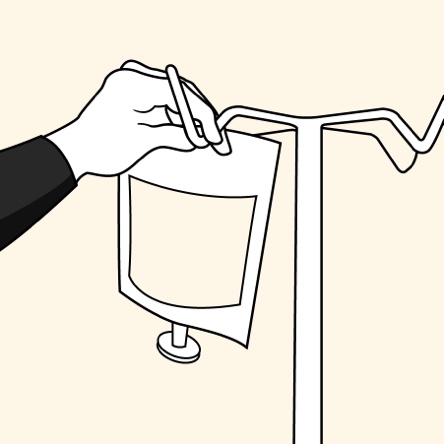
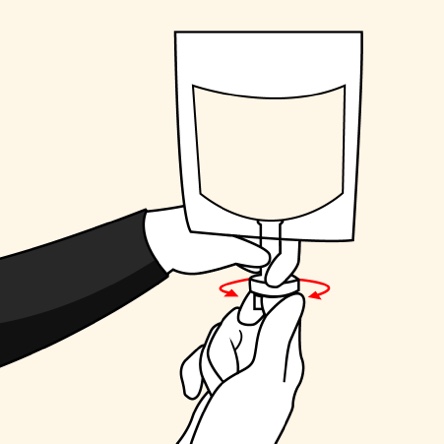
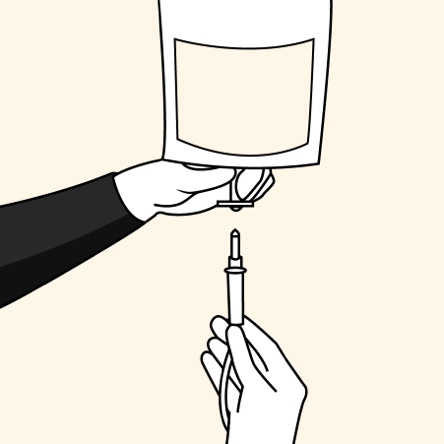
The convenience of an ALBUTEIN FlexBag (albumin [human] U.S.P.)1,2
- No requirement for vented infusion sets of filters
- Flexible container for convenient storage and portability
- Durable and easy-to-spike port designed to avoid needle sticks
- No preservatives, polyvinyl chloride (PVC), diethylhexy phthalate (DEHP), or other plasticizers
- Easy-to-remove twist-off cap
- Easy-to-open protective overwrap
- Latex-free
- Stable for 3 years when stored below 30°C
- Protect from freezing
Administer ALBUTEIN FlexBags in 4 simple steps1
Do not use bags in series connections. Such use could result in air embolism due to residual air being drawn from the primary bag before the administration of the fluid from the secondary bag is complete.

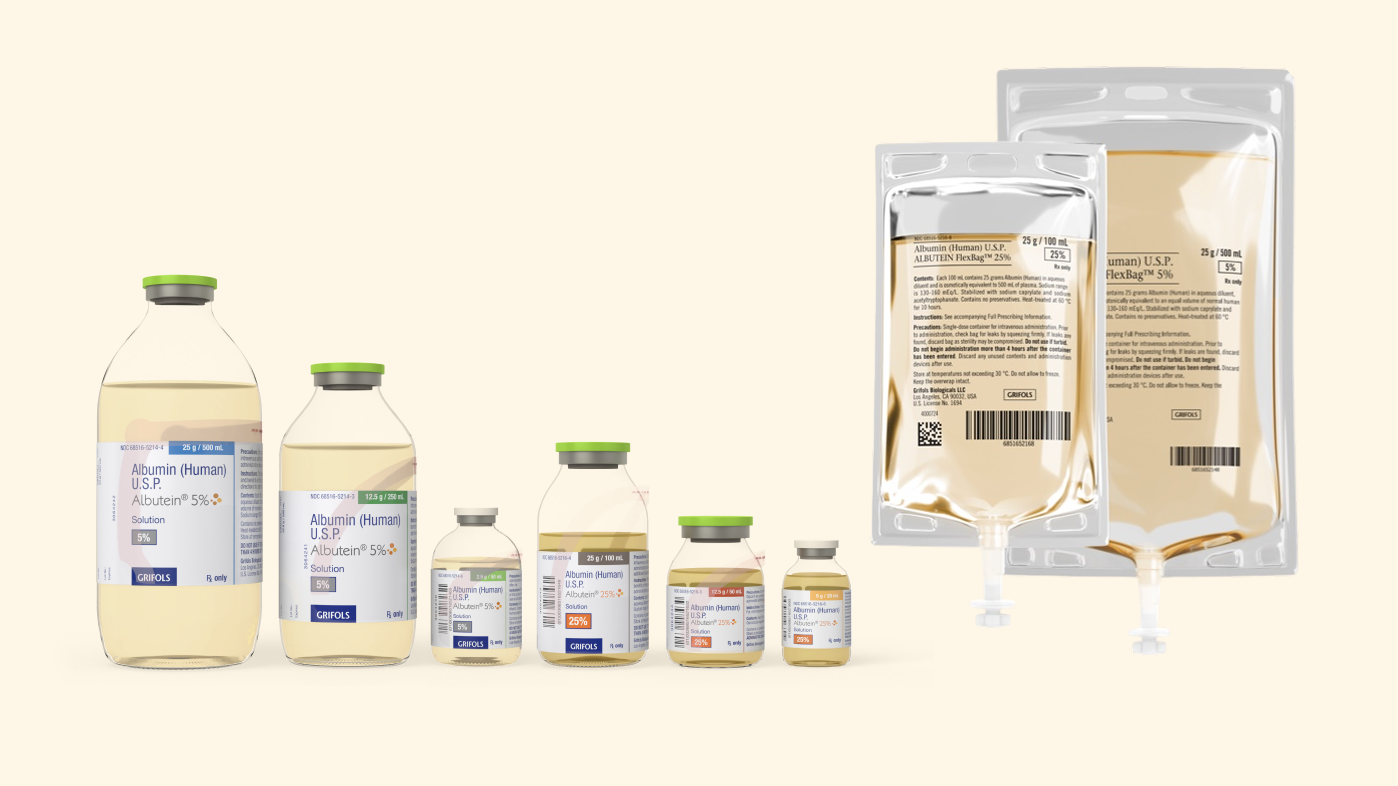
The first and only 5% albumin available in a 500 mL bag1
ALBUTEIN 5%, available in FlexBag sizes:
- 250 mL
- 500 mL
ALBUTEIN 25%, available in FlexBag sizes:
- 50 mL
- 100 mL
Order ALBUTEIN
ALBUTEIN 5% and 25% are available to order in the easy-to-use FlexBags or vials
Have a question about ALBUTEIN or need more information?
Get in touch with a Grifols representative today
Important Safety Information
ALBUTEIN® 5% (albumin [human] U.S.P.) is indicated for: hypovolemia, cardiopulmonary bypass procedures, hypoalbuminemia, and plasma exchange.
ALBUTEIN® 25% (albumin [human] U.S.P.) is indicated for: hypovolemia, cardiopulmonary bypass procedures, acute nephrosis, hypoalbuminemia, ovarian hyperstimulation syndrome, neonatal hyperbilirubinemia, adult respiratory distress syndrome (ARDS), and prevention of central volume depletion after paracentesis due to cirrhotic ascites.
ALBUTEIN 5% and 25% are contraindicated in patients with a history of hypersensitivity to albumin preparations or to any of the excipients, and in patients with severe anemia or cardiac failure with normal or increased intravascular volume.
Allergic or anaphylactic reactions require immediate discontinuation of the infusion and implementation of appropriate medical treatment.
Hypervolemia may occur if the dosage and rate of infusion are not adjusted to the patient’s volume status. At the first clinical signs of fluid overload, the infusion must be slowed or stopped immediately. Use albumin with caution in conditions where hypervolemia and its consequences or hemodilution could represent a special risk to the patient.
The colloid-osmotic effect of human albumin 25% is approximately five times that of blood plasma. Therefore, when concentrated albumin is administered, care must be taken to assure adequate hydration of the patient. Patients should be monitored carefully to guard against circulatory overload and hyperhydration. Patients with marked dehydration require administration of additional fluids.
Concentrated (20% - 25%) human albumin solutions are relatively low in electrolytes compared to 4% - 5% human albumin solutions. Regularly monitor the electrolyte status of the patient and take appropriate steps to restore or maintain the electrolyte balance when albumin is administered.
Regular monitoring of coagulation and hematology parameters is necessary if comparatively large volumes are to be replaced. Care must be taken to ensure adequate substitution of other blood constituents (coagulation factors, electrolytes, platelets and erythrocytes).
Regularly monitor hemodynamic parameters during administration of ALBUTEIN 5% and 25% (albumin [human] U.S.P.).
ALBUTEIN 5% and 25% must not be diluted with sterile water for injection as this may cause hemolysis in recipients.
Albumin is a derivative of human blood. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of viral diseases. A theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD) is also considered extremely remote. No cases of transmission of viral diseases or CJD have ever been identified for ALBUTEIN 5% or 25%.
The most serious adverse reactions with use of albumin are anaphylactic shock, heart failure and pulmonary edema. The most common adverse reactions are anaphylactoid type reactions. Adverse reactions to ALBUTEIN normally resolve when the infusion rate is slowed or the infusion is stopped. In case of severe reactions, the infusion should be stopped and appropriate treatment initiated.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please see full Prescribing Information for ALBUTEIN FlexBag 5%, ALBUTEIN FlexBag 25%, ALBUTEIN vial 5% and ALBUTEIN vial 25%
References
- ALBUTEIN FlexBag® 5% (albumin [human] U.S.P.) Prescribing Information. Grifols.
- ALBUTEIN FlexBag® 25% (albumin [human] U.S.P.) Prescribing Information. Grifols.