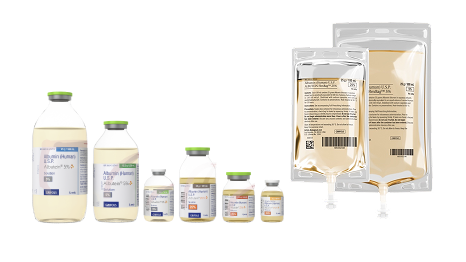
ALBUTEIN® is more convenient than ever
ALBUTEIN (albumin [human] U.S.P.) in 5% and 25% concentrations is now available in a flexible, easy-to-use bag1,2

ALBUTEIN 5% is indicated for hypovolemia, cardiopulmonary bypass procedures, hypoalbuminemia, and plasma exchange.
ALBUTEIN 25% is indicated for hypovolemia, cardiopulmonary bypass procedures, acute nephrosis, hypoalbuminemia, ovarian hyperstimulation syndrome, neonatal hyperbilirubinemia, adult respiratory distress syndrome (ARDS), and prevention of central volume depletion after paracentesis due to cirrhotic ascites.

Administer with ease
Designed to provide improved convenience, storage, and administration of albumin 5% and 25%, available in multiple sizes1,2
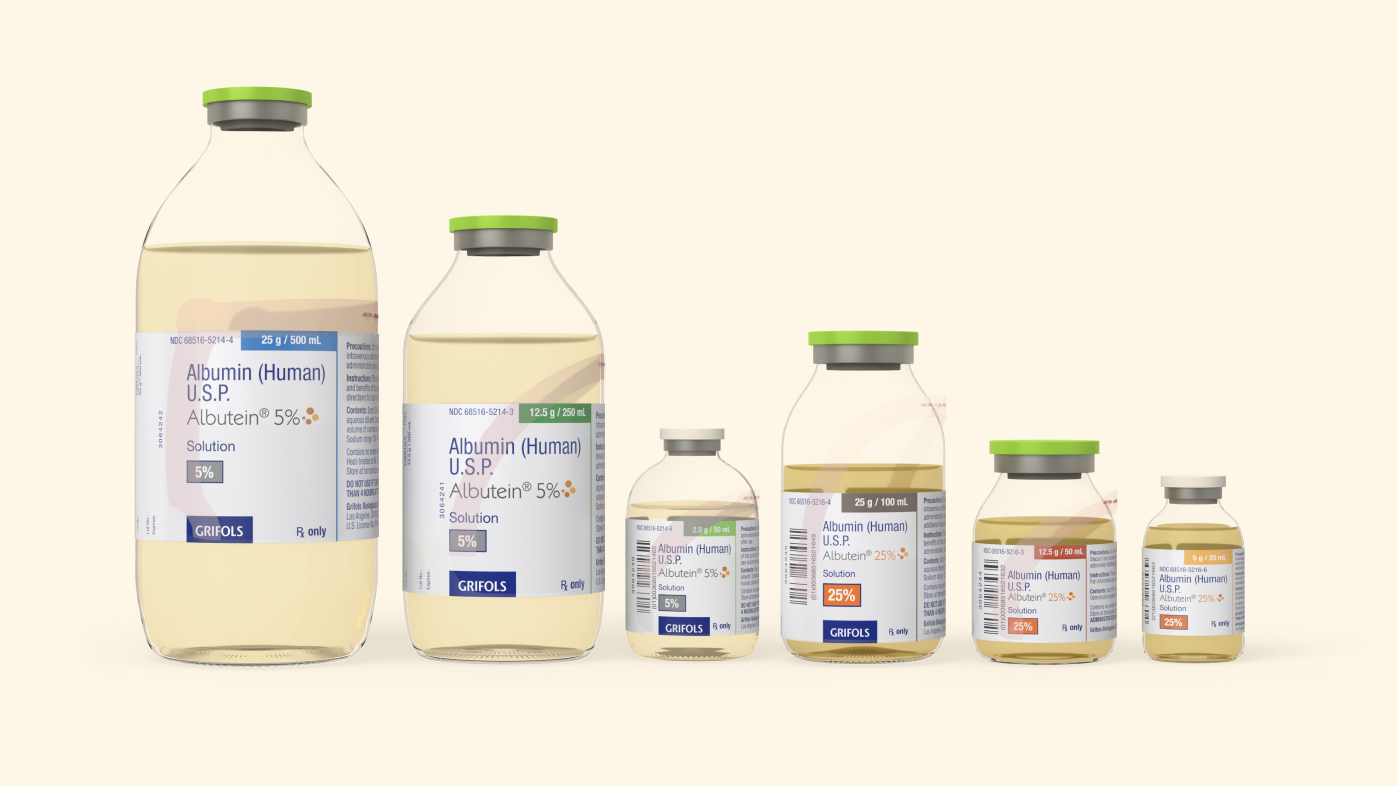
Versatile sizes
ALBUTEIN 5% and 25% vials offer another option from Grifols for your clinical needs3,4
Explore albumin
Albumin is the most abundant plasma protein in the body, comprising more than half of the total protein in human plasma1
Have a question about ALBUTEIN or need more information?
Get in touch with a Grifols representative today
Your health, our commitment
Grifols is a leading global healthcare company founded in Barcelona in 1909 that develops and provides plasma-derived medicines and other innovative biopharmaceuticals to enable millions of patients around the world to lead more productive lives. The company provides its innovative healthcare solutions and services in more than 110 countries and regions.
For more information about ALBUTEIN® or other Grifols products, request to speak with a Grifols Hospital Specialty Representative, or please visit www.grifols.com

Important Safety Information
ALBUTEIN® 5% (albumin [human] U.S.P.) is indicated for: hypovolemia, cardiopulmonary bypass procedures, hypoalbuminemia, and plasma exchange.
ALBUTEIN® 25% (albumin [human] U.S.P.) is indicated for: hypovolemia, cardiopulmonary bypass procedures, acute nephrosis, hypoalbuminemia, ovarian hyperstimulation syndrome, neonatal hyperbilirubinemia, adult respiratory distress syndrome (ARDS), and prevention of central volume depletion after paracentesis due to cirrhotic ascites.
ALBUTEIN 5% and 25% are contraindicated in patients with a history of hypersensitivity to albumin preparations or to any of the excipients, and in patients with severe anemia or cardiac failure with normal or increased intravascular volume.
Allergic or anaphylactic reactions require immediate discontinuation of the infusion and implementation of appropriate medical treatment.
Hypervolemia may occur if the dosage and rate of infusion are not adjusted to the patient’s volume status. At the first clinical signs of fluid overload, the infusion must be slowed or stopped immediately. Use albumin with caution in conditions where hypervolemia and its consequences or hemodilution could represent a special risk to the patient.
The colloid-osmotic effect of human albumin 25% is approximately five times that of blood plasma. Therefore, when concentrated albumin is administered, care must be taken to assure adequate hydration of the patient. Patients should be monitored carefully to guard against circulatory overload and hyperhydration. Patients with marked dehydration require administration of additional fluids.
Concentrated (20% - 25%) human albumin solutions are relatively low in electrolytes compared to 4% - 5% human albumin solutions. Regularly monitor the electrolyte status of the patient and take appropriate steps to restore or maintain the electrolyte balance when albumin is administered.
Regular monitoring of coagulation and hematology parameters is necessary if comparatively large volumes are to be replaced. Care must be taken to ensure adequate substitution of other blood constituents (coagulation factors, electrolytes, platelets and erythrocytes).
Regularly monitor hemodynamic parameters during administration of ALBUTEIN 5% and 25% (albumin [human] U.S.P.).
ALBUTEIN 5% and 25% must not be diluted with sterile water for injection as this may cause hemolysis in recipients.
Albumin is a derivative of human blood. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of viral diseases. A theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD) is also considered extremely remote. No cases of transmission of viral diseases or CJD have ever been identified for ALBUTEIN 5% or 25%.
The most serious adverse reactions with use of albumin are anaphylactic shock, heart failure and pulmonary edema. The most common adverse reactions are anaphylactoid type reactions. Adverse reactions to ALBUTEIN normally resolve when the infusion rate is slowed or the infusion is stopped. In case of severe reactions, the infusion should be stopped and appropriate treatment initiated.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please see full Prescribing Information for ALBUTEIN FlexBag 5%, ALBUTEIN FlexBag 25%, ALBUTEIN vial 5% and ALBUTEIN vial 25%
References
- ALBUTEIN FlexBag®5% (albumin [human] U.S.P.) Prescribing Information. Grifols.
- ALBUTEIN FlexBag® 25% (albumin [human] U.S.P.) Prescribing Information. Grifols.
- ALBUTEIN® 5% vial (albumin [human] U.S.P.) Prescribing Information. Grifols.
- ALBUTEIN® 25% vial (albumin [human] U.S.P.) Prescribing Information. Grifols.